Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kai, Takeshi; Toigawa, Tomohiro; Matsuya, Yusuke; Hirata, Yuho; Tezuka, Tomoya*; Tsuchida, Hidetsugu*; Yokoya, Akinari*
RSC Advances (Internet), 13(46), p.32371 - 32380, 2023/11
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)Although scientific knowledge of photolysis and radiolysis of water is widely used in the life sciences and other fields, the formation mechanism of the spatial distribution of hydrated electrons (spur) resulting from energy deposition to water is still not well understood. The chemical reaction times of hydrated electrons, OH radicals, and H O
O in the spur strongly depend on the spur radius. In our previous study, we elucidated the mechanism at a specific given energy (12.4 eV) by first-principles calculations. In the present study, we performed first-principles calculations of the spur radius at the deposition energies of 11-19 eV. The calculated spur radius is 3-10 nm, which is consistent with the experimental prediction (~4 nm) for the energy range of 8-12.4 eV, and the spur radius gradually increases with increasing energy. The spur radius is a new scientific knowledge and is expected to be widely used for estimating radiation DNA damage.
in the spur strongly depend on the spur radius. In our previous study, we elucidated the mechanism at a specific given energy (12.4 eV) by first-principles calculations. In the present study, we performed first-principles calculations of the spur radius at the deposition energies of 11-19 eV. The calculated spur radius is 3-10 nm, which is consistent with the experimental prediction (~4 nm) for the energy range of 8-12.4 eV, and the spur radius gradually increases with increasing energy. The spur radius is a new scientific knowledge and is expected to be widely used for estimating radiation DNA damage.
Kumagai, Yuta
Hoshasen Kagaku (Internet), (115), p.43 - 49, 2023/04
Oxidation and dissolution of uranium oxide materials has been a subject of numerous studies as a basis of the geological disposal technology for spent nuclear fuel. The understandings obtained by these studies provide useful suggestions for research and development regarding the retrieval and storage of nuclear fuel debris generated by a nuclear severe accident. Here, these research backgrounds of oxidative dissolution of uranium oxides are briefly reviewed and some studies relating to radiation-induced reactions will be introduced.
Kai, Takeshi; Toigawa, Tomohiro; Ukai, Masatoshi*; Fujii, Kentaro*; Watanabe, Ritsuko*; Yokoya, Akinari*
Journal of Chemical Physics, 158(16), p.164103_1 - 164103_8, 2023/04
New insight into water radiolysis and photolysis is indispensable in the dramatic progress of sciences and technologies in various research areas. In the radiation field, reactive hydrated electrons are considerably produced along radiation tracks. Although the formation results from a transient dynamic correlation between ejected electrons and water, the individual mechanisms of electron thermalization, delocalization, and polarization are unknown. Using a dynamic Monte Carlo code, we show herein that the ejected electrons are immediately delocalized by molecular excitations in parallel with phonon polarization and gradually thermalized by momentum transfer with an orientation polarization in a simultaneous manner. Our results show that these mechanisms heavily depend on the intermolecular vibration and rotation modes peculiar to water. We expect our approach to be a powerful technique for connecting physical and chemical processes in various solvents.
Kai, Takeshi; Toigawa, Tomohiro; Matsuya, Yusuke*; Hirata, Yuho; Tezuka, Tomoya*; Tsuchida, Hidetsugu*; Yokoya, Akinari*
RSC Advances (Internet), 13(11), p.7076 - 7086, 2023/03
Times Cited Count:3 Percentile:81.33(Chemistry, Multidisciplinary)Scientific insights of water radiolysis are widely used in the life sciences and so on, however, the formation mechanism of radicals, a product of water radiolysis, is still not well understood. We are challenging to develop a simulation code to solve this formation mechanism from the viewpoint of radiation physics. Our first-principles calculations have revealed that the behavior of secondary electrons in water is governed not only by collisional effects but also by polarization effects. Furthermore, from the predicted ratio of ionization to electronic excitation, based on the spatial distribution of secondary electrons, we successfully reproduce the initial yield of hydrated electrons predicted in terms of radiation chemistry. The code provides us a reasonable spatiotemporal connection from radiation physics to radiation chemistry. Our findings are expected to provide newly scientific insights for understanding the earliest stages of water radiolysis.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
 mesocrystal formation
mesocrystal formationLi, Z.*; Piankova, D.*; Yang, Y.*; Kumagai, Yuta; Zschiesche, H.*; Jonsson, M.*; Tarakina, N. V.*; Soroka, I. L.*
Angewandte Chemie; International Edition, 61(6), p.e202112204_1 - e202112204_9, 2022/02
Times Cited Count:4 Percentile:30.82(Chemistry, Multidisciplinary)The role of intermediate phases in CeO mesocrystal formation from aqueous Ce(III) solutions subjected to
mesocrystal formation from aqueous Ce(III) solutions subjected to  -radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO
-radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO
mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
Kumagai, Yuta; Kimura, Atsushi*; Taguchi, Mitsumasa*; Watanabe, Masayuki
Radiation Physics and Chemistry, 191, p.109831_1 - 109831_8, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)In this study, we investigated and compared the effects of a high-silica zeolite (HMOR) on the radiation-induced degradation of three aromatic chlorides, 2-chlorophenol (2-ClPh), 2-chloroaniline (2-ClAn), and 2-chlorobenzoic acid (2-ClBA), in order to examine its potential to reduce the influence of ions in water matrix in the irradiation treatment of water-soluble organic compounds. In the presence of ions reactive to radicals, the degradation of 2-ClPh in water was inhibited, but the combined use of HMOR much improved the degradation yield. This improvement was attributed to high performance of HMOR in adsorption of 2-ClPh. Similarly, HMOR was effective for adsorption of 2-ClAn and facilitated the 2-ClAn degradation by irradiation. In contrast, HMOR was poor at adsorption of 2-ClBA and consistently the degradation of 2-ClBA in the water-HMOR mixture was inhibited by the radical scavenger. These results demonstrate that HMOR can mitigate the influence of radical scavengers in water.
Hata, Kuniki
Zairyo To Kankyo, 70(12), p.468 - 473, 2021/12
In order to estimate corrosive environment in the contaminated water at Fukushima Daiichi Nuclear Power Station, effects of oxidants, such as H O
O , which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and
, which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and  -radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
-radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.

Miyazaki, Yasunori; Adachi, Junichi*; Masuda, Ryotaro*; Gejo, Tatsuo*; Hoshino, Masamitsu*
Photon Factory Activity Report 2021 (Internet), 2 Pages, 2021/00
no abstracts in English
 -radiation and H
-radiation and H O
O induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acid
induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acidKumagai, Yuta; Jonsson, M.*
Dalton Transactions (Internet), 49(6), p.1907 - 1914, 2020/02
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO . Using phthalic acid as a model compound, we have measured adsorption on UO
. Using phthalic acid as a model compound, we have measured adsorption on UO and investigated effects on the reaction between H
and investigated effects on the reaction between H O
O and UO
and UO and on oxidative dissolution induced by
and on oxidative dissolution induced by  -irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H
-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H O
O and UO
and UO in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO
in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO surface, it is not involved in the interfacial reaction by H
surface, it is not involved in the interfacial reaction by H O
O . In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H
. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H O
O generated by radiolysis was consumed by UO
generated by radiolysis was consumed by UO . The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO
. The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO .
.
Hata, Kuniki; Inoue, Hiroyuki*
Journal of Nuclear Science and Technology, 56(9-10), p.842 - 850, 2019/09
Times Cited Count:1 Percentile:11.15(Nuclear Science & Technology)To investigate the effect of dissolved species from steels on the radiolysis processes of Cl , radiolysis simulations of solutions containing both Cl
, radiolysis simulations of solutions containing both Cl and Fe
and Fe were carried out. The results showed that the generation of radiolytic products (H
were carried out. The results showed that the generation of radiolytic products (H O
O , O
, O and H
and H ) increased mainly by the addition of Fe
) increased mainly by the addition of Fe , and a drop in the pH was caused by the hydrolysis of Fe
, and a drop in the pH was caused by the hydrolysis of Fe . This pH drop enhanced the reactivity of Cl
. This pH drop enhanced the reactivity of Cl with
with  OH, which induced additional generation of H
OH, which induced additional generation of H O
O and O
and O . These results show that low concentrations of Cl
. These results show that low concentrations of Cl (1
(1  10
10 mol/dm
mol/dm = 35ppm) in the presence of Fe
= 35ppm) in the presence of Fe could influence the generation of H
could influence the generation of H O
O and O
and O during water radiolysis. However, it is considered that these effects of Fe
during water radiolysis. However, it is considered that these effects of Fe and low concentration of Cl
and low concentration of Cl on water radiolysis are less important for corrosion of steels due to the low concentrations of H
on water radiolysis are less important for corrosion of steels due to the low concentrations of H O
O and O
and O generated. The other process, such as dissolution of iron enhanced by FeOOH, might predominantly induce corrosion under the conditions of solutions with low concentrations of H
generated. The other process, such as dissolution of iron enhanced by FeOOH, might predominantly induce corrosion under the conditions of solutions with low concentrations of H O
O and O
and O .
.
 induced by H
induced by H O
O and
and  -irradiation
-irradiationKumagai, Yuta; Fidalgo, A. B.*; Jonsson, M.*
Journal of Physical Chemistry C, 123(15), p.9919 - 9925, 2019/04
Times Cited Count:19 Percentile:62.54(Chemistry, Physical)Radiation-induced oxidative dissolution of uranium dioxide (UO ) is one of the most important chemical processes of U driven by redox reactions. We have examined the effect of UO
) is one of the most important chemical processes of U driven by redox reactions. We have examined the effect of UO stoichiometry on the oxidative dissolution of UO
stoichiometry on the oxidative dissolution of UO induced by hydrogen peroxide (H
induced by hydrogen peroxide (H O
O ) and
) and  -ray irradiation. By comparing the reaction kinetics of H
-ray irradiation. By comparing the reaction kinetics of H O
O between stoichiometric UO
between stoichiometric UO and hyper-stoichiometric UO
and hyper-stoichiometric UO , we observed a significant difference in reaction speed and U dissolution kinetics. The stoichiometric UO
, we observed a significant difference in reaction speed and U dissolution kinetics. The stoichiometric UO reacted with H
reacted with H O
O much faster than the hyper-stoichiometric UO
much faster than the hyper-stoichiometric UO . The U dissolution from UO
. The U dissolution from UO was initially much lower than that from UO
was initially much lower than that from UO , but gradually increased as the oxidation by H
, but gradually increased as the oxidation by H O
O proceeded. The
proceeded. The  -ray irradiation induced the U dissolution that is analogous to the kinetics by the exposure to a low concentration (0.2 mM) of H
-ray irradiation induced the U dissolution that is analogous to the kinetics by the exposure to a low concentration (0.2 mM) of H O
O . The exposure to higher H
. The exposure to higher H O
O concentrations caused lower U dissolution and resulted in deviation from the U dissolution behavior by
concentrations caused lower U dissolution and resulted in deviation from the U dissolution behavior by  -ray irradiation.
-ray irradiation.
Hirade, Tetsuya
JPS Conference Proceedings (Internet), 25, p.011022_1 - 011022_3, 2019/03
OH radicals which are very reactive are formed by radiation decomposition in water. The behavior of OH radicals is important in corrosion of materials and reactions in living bodies. Recently, the reaction occurring between positronium (Ps) formed by OH radicals formed at the end of the positron track when positron is incident and positronium (Ps) formed by reaction of excess electrons formed with OH radical formation with the thermo-positron, it is reported that quantum beat occurs due to spin correlation. This quantum beat seems to have a period depending on the hyperfine coupling constant of OH radical. It is thought that the period and intensity of the quantum beat depends on the temperature, and it seems that it reflects the state around the OH radical. From the temperature dependence of the quantum beat detected by the reaction of this spin-correlated OH radical and triplet positronium we will explain what the liquid structure might be.
 O
O and UO
and UO
Fidalgo, A. B.*; Kumagai, Yuta; Jonsson, M.*
Journal of Coordination Chemistry, 71(11-13), p.1799 - 1807, 2018/07
Times Cited Count:29 Percentile:88.5(Chemistry, Inorganic & Nuclear)In this work, we have studied the reaction between H O
O and UO
and UO with particular focus on the nature of the hydroxyl radical formed as an intermediate. Experiments were performed to study the kinetics of the reaction at different initial H
with particular focus on the nature of the hydroxyl radical formed as an intermediate. Experiments were performed to study the kinetics of the reaction at different initial H O
O concentrations. The results show that the consumption rates at a given H
concentrations. The results show that the consumption rates at a given H O
O concentration are different depending on the initial H
concentration are different depending on the initial H O
O concentration. This is attributed to an alteration of the reactive interface, likely caused by blocking of surface sites by oxidized U/surface-bound hydroxyl radicals. The U dissolution yield decreases with increasing initial H
concentration. This is attributed to an alteration of the reactive interface, likely caused by blocking of surface sites by oxidized U/surface-bound hydroxyl radicals. The U dissolution yield decreases with increasing initial H O
O concentration. This is expected from the mechanism of catalytic decomposition of H
concentration. This is expected from the mechanism of catalytic decomposition of H O
O on oxide surfaces. As the experiments were performed in solutions containing 10 mM and a strong concentration dependence was observed in the 0.2 - 2.0 mM H
on oxide surfaces. As the experiments were performed in solutions containing 10 mM and a strong concentration dependence was observed in the 0.2 - 2.0 mM H O
O concentration range, we conclude that the intermediate hydroxyl radical is surface bound rather than free.
concentration range, we conclude that the intermediate hydroxyl radical is surface bound rather than free.
Sato, Junya; Kikuchi, Hiroshi*; Kato, Jun; Sakakibara, Tetsuro; Matsushima, Ryotatsu; Sato, Fuminori; Kojima, Junji; Nakazawa, Osamu
QST-M-8; QST Takasaki Annual Report 2016, P. 62, 2018/03
no abstracts in English
Taguchi, Mitsumasa*; Kumagai, Yuta; Kimura, Atsushi*
IAEA-TECDOC-1855, p.106 - 116, 2018/00
The technology for the decomposition of trace amounts of halogenated pharmaceuticals/antibiotics was developed in wastewater by use of the combination method of zeolite adsorbent and ionizing radiation. HMOR, a hydrophobic high-silica mordenite-type zeolite, was employed to concentrate 2-chlorophenol (2-ClPh) as a simple model of halogenated pharmaceuticals/antibiotics. HMOR adsorbed above 99% of 2-ClPh from dilute aqueous solutions. The yield of Cl production in HMOR mixture corresponded to the aqueous solution containing 10 fold higher concentration of dissolved 2-ClPh. Clofibrate and triclosan, one of chlorinated pharmaceuticals/antibiotics, in real wastewater were treated by use of the combination method of HMOR and ionizing radiation. Production yield of Cl
production in HMOR mixture corresponded to the aqueous solution containing 10 fold higher concentration of dissolved 2-ClPh. Clofibrate and triclosan, one of chlorinated pharmaceuticals/antibiotics, in real wastewater were treated by use of the combination method of HMOR and ionizing radiation. Production yield of Cl by use of the adsorbent was about twice higher than that in aqueous solution, and HMOR was contributed for effective reduction of chlorinated pharmaceuticals/antibiotics in real wastewater.
by use of the adsorbent was about twice higher than that in aqueous solution, and HMOR was contributed for effective reduction of chlorinated pharmaceuticals/antibiotics in real wastewater.
Watanabe, Ritsuko*; Kai, Takeshi; Hattori, Yuya*
Radioisotopes, 66(11), p.525 - 530, 2017/11
To understand the mechanisms of radiation biological effects, modeling and simulation studies are important. In particular, simulation approach is powerful tool to evaluate modeling of mechanisms and the relationship among experimental results in different spatial scale of biological systems such as DNA molecular and cell. This article summarizes our approach to evaluate radiation action on DNA and cells by combination of knowledge in radiation physics, chemistry and biology. It contains newly theoretical approach to estimate physico-chemical process of DNA damage induction in addition to typical method of DNA damage prediction. Outline of the mathematical model for dynamics of DNA damage and cellular response is also presented.
Nagaishi, Ryuji
Radioisotopes, 66(11), p.601 - 610, 2017/11
no abstracts in English
 and Br
and Br and its effect on corrosion of a low-alloy steel
and its effect on corrosion of a low-alloy steelHata, Kuniki; Inoue, Hiroyuki*; Kojima, Takao*; Kasahara, Shigeki; Hanawa, Satoshi; Ueno, Fumiyoshi; Tsukada, Takashi; Iwase, Akihiro*
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2017 (AWC 2017) (USB Flash Drive), p.304 - 314, 2017/09
A model simulation of  radiolysis of mixed solutions of NaCl and NaBr was carried out. The simulation result agreed well with the experimental result, and Br
radiolysis of mixed solutions of NaCl and NaBr was carried out. The simulation result agreed well with the experimental result, and Br played an important role in determining the amounts of products from water radiolysis. The simulation result also showed that, in highly pure NaCl solutions, the steady-state concentration of a radolytic product, H
played an important role in determining the amounts of products from water radiolysis. The simulation result also showed that, in highly pure NaCl solutions, the steady-state concentration of a radolytic product, H O
O , was mainly controlled by three reactions (Cl
, was mainly controlled by three reactions (Cl +
+  OH
OH  ClOH
ClOH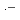 , ClOH
, ClOH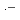
 Cl
Cl +
+  OH, and ClOH
OH, and ClOH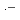 + H
+ H
 Cl
Cl + H
+ H O), which indicated that accurate evaluation of the rate constants of these reactions was very important in improving the radiolysis simulation of solutions containing Cl
O), which indicated that accurate evaluation of the rate constants of these reactions was very important in improving the radiolysis simulation of solutions containing Cl . An immersion test using a low-alloy steel, SQV2A, in the mixed solutions was also carried out under irradiation. The corrosion rate increased or decreased depending on the pH or the concentrations of the halide ions in a similar way to the change in concentration of H
. An immersion test using a low-alloy steel, SQV2A, in the mixed solutions was also carried out under irradiation. The corrosion rate increased or decreased depending on the pH or the concentrations of the halide ions in a similar way to the change in concentration of H O
O produced from water radiolysis, which is affected by the presence of Cl
produced from water radiolysis, which is affected by the presence of Cl and Br
and Br . However, at high pH values (
. However, at high pH values ( 12), the corrosion rate was almost zero even though the concentration of H
12), the corrosion rate was almost zero even though the concentration of H O
O was high. This could be attributed to enhancement of the passivity of test specimens at higher pH values.
was high. This could be attributed to enhancement of the passivity of test specimens at higher pH values.
 -ray irradiation from different types of zeolites in aqueous solution
-ray irradiation from different types of zeolites in aqueous solutionKumagai, Yuta; Kimura, Atsushi*; Taguchi, Mitsumasa*; Watanabe, Masayuki
Journal of Physical Chemistry C, 121(34), p.18525 - 18533, 2017/08
Times Cited Count:7 Percentile:26.48(Chemistry, Physical)H production by irradiation of zeolite-water mixtures was studied, to investigate effect of zeolites in the reaction process for H
production by irradiation of zeolite-water mixtures was studied, to investigate effect of zeolites in the reaction process for H . Four different types of zeolites were examined comparatively under anoxic and under aerated conditions. High production yields of H
. Four different types of zeolites were examined comparatively under anoxic and under aerated conditions. High production yields of H were observed for the zeolites of high Al contents at low water fraction and under anoxic condition, compared to zeolites having lower Al contents. A comparison of the H
were observed for the zeolites of high Al contents at low water fraction and under anoxic condition, compared to zeolites having lower Al contents. A comparison of the H yields in connection with chemical analysis of the zeolites suggests that extraframework Al species in the zeolites are involved in a reaction pathway for H
yields in connection with chemical analysis of the zeolites suggests that extraframework Al species in the zeolites are involved in a reaction pathway for H . Meanwhile, under aerated condition and at high mixing ratio of water, the difference in H
. Meanwhile, under aerated condition and at high mixing ratio of water, the difference in H yield among the zeolites was suppressed and the yields of H
yield among the zeolites was suppressed and the yields of H were lower than those under anoxic condition probably due to H
were lower than those under anoxic condition probably due to H O
O produced by water radiolysis. The comparable H
produced by water radiolysis. The comparable H yields suggest another reaction pathway for H
yields suggest another reaction pathway for H which is less dependent on the structure and composition of the zeolites.
which is less dependent on the structure and composition of the zeolites.